Pharmaceutical Glass Packaging Market Forecast to Reach USD 14.2 Billion by 2036, Driven by Drug Safety Requirements
US pharmaceutical glass packaging sales grow 5.4% CAGR, driven by drug production expansion, biopharma use, specialty packaging, and supply chain reliability.
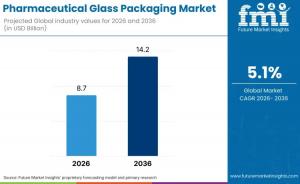
NEWARK, DE, UNITED STATES, January 29, 2026 /EINPresswire.com/ -- The global pharmaceutical glass packaging market is entering a sustained growth phase, underpinned by regulatory mandates, drug safety priorities, and expanding pharmaceutical manufacturing capacity worldwide. According to industry analysis by Future Market Insights (FMI), the market is forecast to reach USD 8.7 billion in 2026 and expand to USD 14.2 billion by 2036, registering a compound annual growth rate (CAGR) of 5.1% over the ten-year period.
Pharmaceutical glass packaging plays a critical role in ensuring drug sterility, chemical compatibility, and long-term stability across injectable, oral, topical, and diagnostic applications. Governments and regulatory authorities worldwide continue to reinforce compliance requirements, making certified glass containers a non-negotiable component of regulated drug manufacturing and distribution.
Request Your Sample Now – Unlock Growth Potential and Discover Key Market Opportunities!
https://www.futuremarketinsights.com/reports/sample/rep-gb-19514
Market Context: Why Pharmaceutical Glass Packaging Matters
Pharmaceutical glass containers are subject to rigorous testing and certification before they can be manufactured, imported, sold, or used in regulated drug applications. Regulatory frameworks focus on patient safety, material inertness, and performance consistency, ensuring that glass packaging does not interact with pharmaceutical compounds or compromise therapeutic efficacy.
Key regulatory requirements include:
• Compliance with pharmacopeial standards, such as USP <660> in the United States and corresponding chapters in the European and Japanese Pharmacopoeias
• Chemical resistance, hydrolytic resistance, and thermal shock testing
• Extractables and leachables limits to protect drug integrity during storage and transport
• Manufacturing under current Good Manufacturing Practices (cGMP)
These requirements have elevated pharmaceutical glass packaging from a commodity input to a regulated, performance-critical component of the pharmaceutical value chain.
Quick Market Facts
• Market Value (2026): USD 8.7 billion
• Forecast Value (2036): USD 14.2 billion
• Forecast CAGR (2026–2036): 5.1%
• Leading Product Type: Vials
• Fastest-Growing Countries: India, China, USA, Germany, Japan
• Key Companies: Schott AG, Gerresheimer AG, West Pharmaceutical Services, Bormioli Pharma, SGD Pharma
Product Segmentation: Vials Lead Global Demand
Among product categories, vials account for 42.3% of total market demand, reflecting their central role in injectable drugs, vaccines, and diagnostic sample collection. Other product segments serve complementary but essential roles across pharmaceutical delivery formats.
Pharmaceutical Glass Packaging Market by Product Type
• Vials: 42.3%
• Bottles: 28.4%
• Syringes: 16.7%
• Ampoules: 8.9%
• Others: 3.7%
Key Takeaways
• Vials dominate injectable drug and vaccine packaging
• Bottles support oral and liquid formulations
• Syringes enable pre-filled and patient-ready drug delivery systems
Application Trends: Injectable Drugs Remain the Priority
Application requirements strongly influence packaging selection, with injectable drugs representing the largest share of demand due to sterility and compatibility requirements.
Pharmaceutical Glass Packaging Market by Application
• Injectable Drugs: 38.9%
• Oral Medications: 31.2%
• Topical Preparations: 18.6%
• Diagnostics: 7.8%
• Others: 3.5%
Injectable drugs require packaging solutions capable of maintaining chemical inertness and sterility under controlled manufacturing and storage conditions, reinforcing the reliance on certified glass containers.
Global Trade Dynamics: Exporters and Importers
Major exporting countries include Germany, the United States, Italy, China, and France, supported by advanced glass manufacturing ecosystems and pharmaceutical-grade production capabilities. Germany remains a leading exporter due to its specialization in pharmaceutical glass technologies.
On the import side, Brazil, Mexico, India, China, and the United States represent key demand centers, driven by expanding pharmaceutical manufacturing capacity and healthcare infrastructure investments.
Regional Growth Outlook: Asia-Pacific Leads Expansion
Demand for pharmaceutical glass packaging is rising globally, with growth concentrated in countries expanding pharmaceutical manufacturing while tightening regulatory oversight.
Country-Level CAGR (2026–2036)
• India: 6.8%
• China: 6.2%
• USA: 5.4%
• Germany: 4.9%
• Japan: 4.6%
In India and China, growth is supported by pharmaceutical export expansion, quality improvement programs, and increasing adoption of regulated packaging solutions compatible with international compliance requirements.
Certification and Compliance Requirements
Certification remains central to supplier selection in pharmaceutical glass packaging. Key requirements include:
• USP <660>, European Pharmacopoeia, and Japanese Pharmacopoeia compliance
• ISO 15378 certification for primary pharmaceutical packaging
• ISO 8362 standards for glass vials and cartridges
• Environmental and occupational certifications such as ISO 14001 and ISO 45001
These standards ensure traceability, process control, and consistent container performance across global supply chains.
Competitive Landscape: Performance Over Volume
Competition in the pharmaceutical glass packaging market centers on container quality, sterilization reliability, chemical compatibility, and technical collaboration rather than volume-driven pricing strategies.
Leading Market Participants
• Schott AG – Integrated pharmaceutical glass solutions with strong chemical precision
• Gerresheimer AG – High-quality containers with comprehensive sterilization systems
• West Pharmaceutical Services – Specialized packaging platforms aligned with regulatory needs
• Bormioli Pharma – Precision glass containers for drug protection
• SGD Pharma – Packaging solutions emphasizing inertness and manufacturing integration
Procurement decisions increasingly prioritize long-term supply reliability, validated performance, and alignment with pharmaceutical manufacturing protocols.
Market Outlook Through 2036
The pharmaceutical glass packaging market is expected to maintain steady, regulation-driven growth through 2036. Expansion is shaped less by packaging volume and more by quality requirements, compliance obligations, and performance validation across pharmaceutical manufacturing and healthcare systems.
Why FMI: https://www.futuremarketinsights.com/why-fmi
Have a Look at Related Research Reports on the Packaging Domain:
Demand for Collapsible Rigid Containers in United Kingdom https://www.futuremarketinsights.com/reports/united-kingdom-collapsible-rigid-containers-market
Glassine Paper Market https://www.futuremarketinsights.com/reports/glassine-paper-market
Deblistering Machines Market https://www.futuremarketinsights.com/reports/deblistering-machines-market
Polyethylene Furanoate (PEF) Barrier Resin Market https://www.futuremarketinsights.com/reports/polyethylene-furanoate-pef-barrier-resin-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.